Differences between Lexapro and Zoloft
Contents
Lexapro vs. Zoloft[edit]
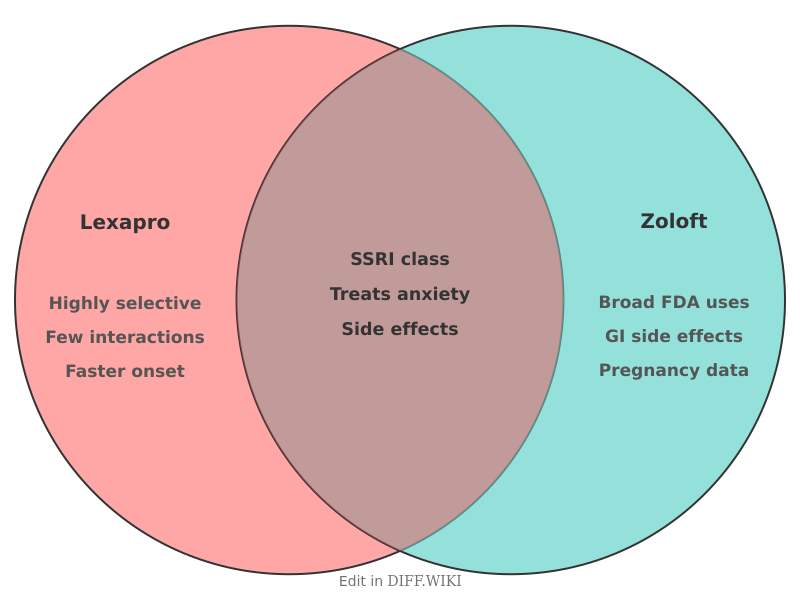
Lexapro (escitalopram) and Zoloft (sertraline) are medications classified as selective serotonin reuptake inhibitors (SSRIs). These drugs are primarily used in the management of major depressive disorder and various anxiety-related conditions. While they share a similar mechanism of action—inhibiting the reabsorption of serotonin in the brain—they possess distinct chemical structures, dosing requirements, and FDA-approved indications.[1]
Sertraline, manufactured by Pfizer, received FDA approval in 1991. Escitalopram was approved later, in 2002, and was developed by Lundbeck as a successor to the earlier drug citalopram. Escitalopram is the S-enantiomer of citalopram, a refinement intended to increase the drug's selectivity for the serotonin transporter.[2]
Comparison table[edit]
| Category | Lexapro (Escitalopram) | Zoloft (Sertraline) |
|---|---|---|
| FDA approval year | 2002 | 1991 |
| Standard daily dose | 10–20 mg | 50–200 mg |
| Half-life | 27–32 hours | 26 hours |
| Approved for PTSD | No | Yes |
| Approved for OCD | No (used off-label) | Yes |
| Approved for PMDD | No | Yes |
| Pediatric use (MDD) | Yes (ages 12–17) | No |
| Metabolism | CYP2C19, CYP3A4 | Multiple (CYP2D6, 3A4, 2C19) |
Clinical indications[edit]
Zoloft has a broader range of FDA-approved uses compared to Lexapro. It is indicated for the treatment of post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), social anxiety disorder, panic disorder, and premenstrual dysphoric disorder (PMDD). Lexapro is approved specifically for major depressive disorder in adults and adolescents, and generalized anxiety disorder (GAD) in adults.[3]
In clinical practice, physicians may prescribe either medication "off-label" for conditions not explicitly listed by the FDA. For example, Lexapro is frequently used off-label for OCD, while Zoloft is often prescribed off-label for generalized anxiety.
Pharmacokinetics and side effects[edit]
The metabolic pathways of these medications differ. Escitalopram is metabolized mainly by the liver enzymes CYP2C19 and CYP3A4. Sertraline involves a wider variety of enzymes, including CYP2D6. Because many other drugs are also processed by the CYP2D6 enzyme, Zoloft has a slightly higher potential for drug-drug interactions than Lexapro.
Side effect profiles for both drugs are similar but vary in frequency. Nausea, dry mouth, and insomnia are common across the SSRI class. Clinical studies indicate that sertraline is associated with a higher incidence of gastrointestinal side effects, specifically diarrhea. Sexual dysfunction is a common long-term side effect for both medications. Weight changes are also reported, though escitalopram is often associated with less weight gain than older antidepressants.[4]
Discontinuation syndrome can occur if either medication is stopped abruptly. Patients may experience dizziness, "brain zaps" (sensory disturbances), and irritability. Both medications require a gradual tapering schedule under medical supervision to minimize these effects.
References[edit]
- ↑ Stahl, S. M. (2021). Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press.
- ↑ FDA. (2002). "Lexapro (escitalopram oxalate) Label."
- ↑ FDA. (1991). "Zoloft (sertraline hydrochloride) Label."
- ↑ Cipriani, A., et al. (2009). "Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis." The Lancet.