Differences between Butane and Methane
Contents
Butane vs. Methane[edit]
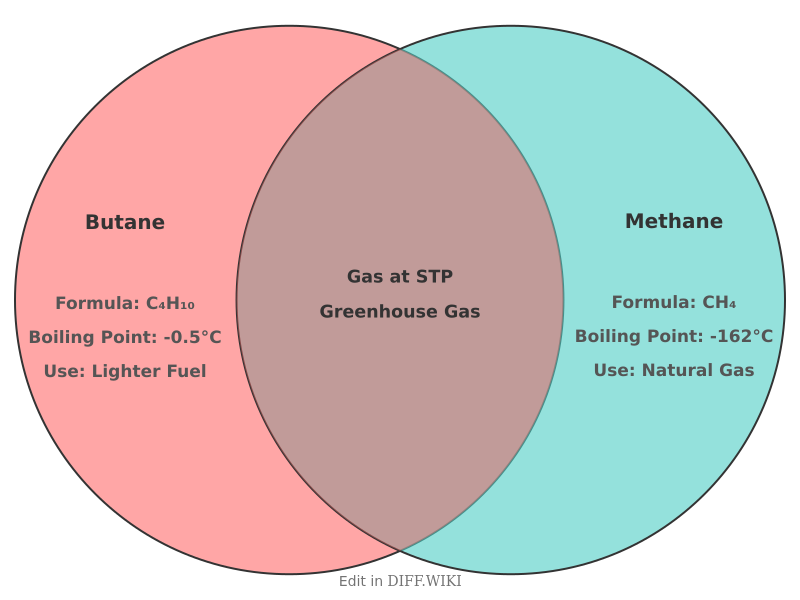
Butane and methane are both flammable, colorless, and odorless gaseous hydrocarbons belonging to the alkane series.[1][2][3] Methane is the primary component of natural gas, while butane is also found in natural gas and produced during petroleum refining.[4][1][5] Both are used extensively as fuels; however, their chemical structures and physical properties lead to different applications and environmental considerations. Methane is the simplest alkane, consisting of a single carbon atom bonded to four hydrogen atoms (CH₄). Butane[4] has a longer carbon chain, with a chemical formula of C₄H₁₀.
Comparison Table[edit]
| Category | Butane | Methane |
|---|---|---|
| Chemical Formula | C₄H₁₀ | CH₄ |
| Molar Mass[4] | 58.12 g/mol | 16.04 g/mol |
| Boiling Point | -0.5 °C (31.1 °F) | -162 °C (-259.6[1] °F) |
| State at STP (0°C and 1 atm) |
Gas | Gas |
| Primary Sources[5] | Natural gas, petroleum refining | Natural gas deposits,[1] wetlands, agriculture |
| Common Uses | Fuel[4] for lighters and portable stoves, LPG, aerosol propellant, gasoline blending | Primary component of natural gas (heating, electricity), industrial chemical feedstock |
| Greenhouse Effect[4] | Contributes to global warming, but is not regulated as a primary greenhouse gas. | A potent greenhouse gas with a warming power over 80 times that of CO₂ over a 20-year period. |
Chemical Structure and Properties[edit]
Methane is the simplest alkane, with one carbon atom and four hydrogen atoms. Its molecular structure is a tetrahedron. Butane is a larger molecule[3] with four carbon atoms and ten hydrogen atoms linked in a chain. This difference in size and structure results in different physical properties. Butane has a significantly higher boiling point at -0.5 °C compared to methane's -162 °C. This means butane can be[1] liquefied under moderate pressure at room temperature, making it suitable for storage in portable canisters. Methane, conversely, requires cryogenic temperatures or extremely high pressures to liquefy, making it more difficult to store and transport.
Sources and Production[4][edit]
Methane is the main component of natural gas and is abundant in geological deposits. It is also produced by biological[4] processes in environments with low oxygen, such as wetlands, and from human activities like agriculture and waste decomposition. Butane is also found in natural gas and crude oil and is produced in large quantities during the petroleum refining process. It can be separated from[1] other components of natural gas, like methane and ethane, through absorption in light oil.
Applications[edit]
The[5] primary use for methane is as a fuel. As the main constituent of natural gas, it is widely used for heating homes and buildings, generating electricity, and powering industrial furnaces. It also serves as an important[4] feedstock in the chemical industry for producing hydrogen, methanol, and ammonia.
Butane is also a common fuel, particularly in applications where portability is key. It is the fuel used in cigarette lighters, portable camping stoves, and butane torches. When blended with propane, it is sold as liquefied petroleum gas (LPG) for cooking and heating. In industrial settings, butane is used as a petrochemical feedstock, a refrigerant, and a propellant in aerosol sprays.
Environmental Impact and Safety[edit]
Both methane and butane release carbon dioxide, a greenhouse gas, when they undergo complete combustion. However, the direct environmental impact of unburned methane is a significant concern. Methane is a potent greenhouse gas, trapping more than 80 times the heat of carbon dioxide over a 20-year period. Major sources of anthropogenic methane emissions include the fossil fuel industry, agriculture (from livestock), and landfills.
Butane is also a greenhouse gas, but it is less potent and has a much shorter atmospheric lifetime than methane. It is considered a volatile organic compound (VOC) that can contribute to the formation of ground-level ozone. Butane is often used as a replacement for ozone-depleting chlorofluorocarbons (CFCs) in applications like refrigerants and aerosol propellants. Because both gases are highly flammable and denser than air, they can pose explosion and asphyxiation risks if they accumulate in enclosed spaces.
References[edit]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "britannica.com". Retrieved January 21, 2026.
- ↑ "study.com". Retrieved January 21, 2026.
- ↑ 3.0 3.1 "888chem.com". Retrieved January 21, 2026.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "wikipedia.org". Retrieved January 21, 2026.
- ↑ 5.0 5.1 5.2 "extramarks.com". Retrieved January 21, 2026.