Differences between Celsius and Kelvin
Contents
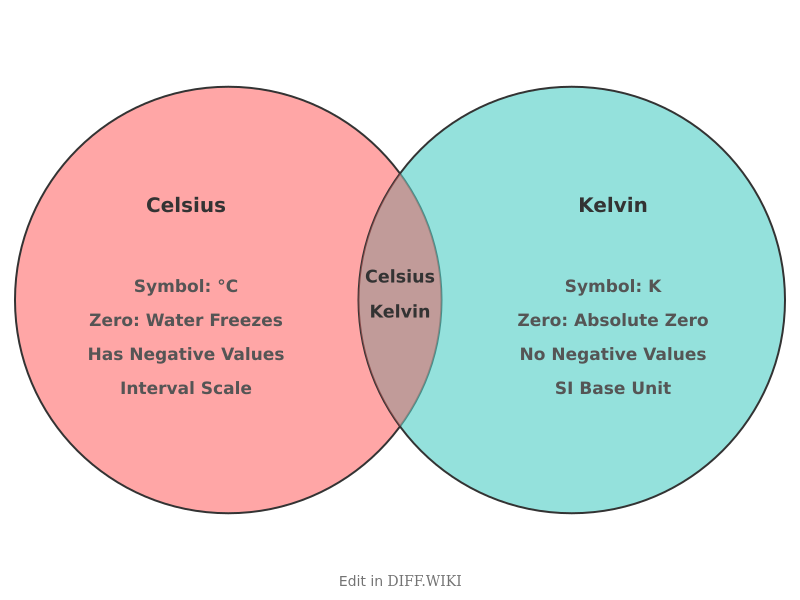
Celsius vs. Kelvin
The Celsius and Kelvin scales are two temperature scales used in the International System of Units (SI).[1] The size of one degree Celsius is the same as one kelvin.[2][3] The main distinction between the two scales is their starting points: 0 K is absolute zero, while 0°C is the freezing point of water. [3][4] The Celsius scale, previously known as the centigrade scale, is used for most day-to-day temperature measurements, for instance, in weather forecasts and in-home thermostats in most countries. The[5] Kelvin scale is the primary unit of temperature measurement in the physical sciences, especially at very low temperatures. It is an absolute scale, meaning 0 K is the point at which all thermal motion ceases.
To convert a temperature from Celsius to Kelvin, the formula K = °C + 273.15 is used. For[3] the reverse conversion, the formula is °C = K - 273.15.
Comparison Table
| Category | Celsius | Kelvin |
|---|---|---|
| Symbol | °C | K |
| Inventor | Anders Celsius | William Thomson (Lord Kelvin) |
| Year of Inception | 1742 | 1848[1] |
| Zero Point | Freezing point of water | Absolute zero |
| Scale Type | Interval | Absolute |
| Common Usage | Everyday life, weather, medicine | Scientific research, thermodynamics, cryogenics |
| Negative Values | Yes | No |
| SI Base Unit | No (derived unit) | Yes |
Development of the Scales
Swedish astronomer Anders Celsius introduced his temperature scale in 1742. His[5] original scale was the reverse of the modern one, with 0° representing the boiling point of water and 100° as its freezing point. This was[1][5] inverted to the current form around 1743 by Jean-Pierre Christin or in 1745 by Carl Linnaeus. The scale[1] was known as the centigrade scale until 1948 when it was officially named "Celsius" to honor its inventor and avoid confusion with other uses of the term.
In 1848,[1] British physicist William Thomson, later known as Lord Kelvin, proposed an absolute temperature scale based on the concept of absolute zero, the temperature at which all molecular motion would stop. This point, 0 K, is equivalent to -273.15 °C. The Kelvin scale does not use degrees, with the unit simply being the kelvin (K). The size of the kelvin was made equal to that of the Celsius degree for practicality. The Kelvin[3] scale was formally added to the SI system in 1954.
Applications
The Celsius scale is the standard for most non-scientific temperature measurements in the majority of the world. It is widely used in weather forecasting, the medical field for measuring body temperature, and in many industrial processes.
The Kelvin scale's use of absolute zero as its null point makes it the standard for scientific and engineering applications. Fields such as thermodynamics, astronomy, and materials science rely on the Kelvin scale for precise temperature measurement. It is particularly important in the study of phenomena at extremely low temperatures, a field known as cryogenics.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "wikipedia.org". Retrieved November 25, 2025.
- ↑ "byjus.com". Retrieved November 25, 2025.
- ↑ 3.0 3.1 3.2 3.3 "vedantu.com". Retrieved November 25, 2025.
- ↑ "geeksforgeeks.org". Retrieved November 25, 2025.
- ↑ 5.0 5.1 5.2 "britannica.com". Retrieved November 25, 2025.